Background: Patient perspectives have shifted towards greater expectations of autonomy and personalized care, leading to growing demands for partnerships between patients, healthcare providers (HCPs), and industry. Such collaborations provide a mechanism to understand the unique needs of patient communities, which is particularly important for rare diseases, such as hereditary hemolytic anemias (HHAs). The Patient Vision Project (initiated and sponsored by Agios Pharmaceuticals) was launched in November 2021, aiming to establish a set of principles for defining industry standards in engagement with multiple stakeholders, including (but not limited to) HCPs, caregivers and patients with HHA.
Methods: The Project consisted of 4 phases:
In-depth discovery (company-wide survey, and one-to-one interviews with patient advocates, HCPs and company leaders), with the goal to understand perspectives on patient-centricity and areas of strength and opportunity to optimize patient engagement
Thematic analysis of discovery findings and recommendations to align on company commitment to develop a patient engagement model
1-Day interactive workshop (patients, HCPs, HHA experts and Agios representatives) for feedback on model
Development of an implementation plan
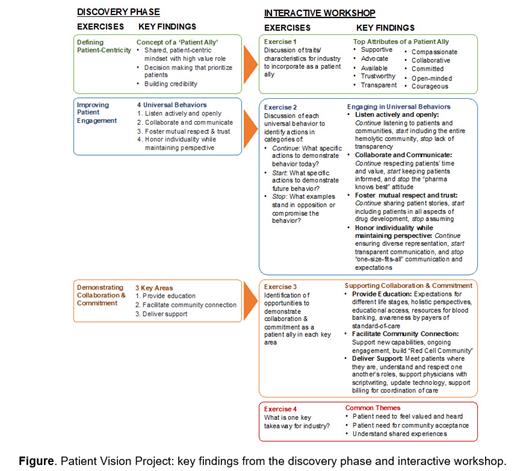
The workshop included 31 participants (patients, HCPs, HHA experts and Agios representatives) divided into breakout groups to define traits of a patient ally, identify universal ally behaviors, discuss ways to support core areas for collaboration and commitment to patients, and provide input to industry on patient needs and expectations (Figure).
Results: During the discovery phase, 116 of 152 (76%) Agios respondents completed the survey, and 7 Agios leaders and 8 HHA experts (HCPs, patients, and patient advocates) were interviewed. Responses to questions pertaining to patient-centricity emphasized patient partnership/prioritization, with 42% of the 152 responses favoring ongoing patient engagement to understand their needs, transparent communication, and identification of collaboration opportunities. On questions related to company engagement with patients, 93% felt connected to patients because of their work, 86% felt they added value, and 86% felt the company had a clear approach for patient engagement. Interviewees emphasized the importance for company leadership to prioritize patient-centricity, as well as the value of hearing the patient voice and meeting their needs, while being transparently connected to their lives, experiences, and priorities. Findings from the discovery phase led to the concept of a “patient ally”, along with 4 universal behaviors to define allyship: (1) Listening actively and openly; (2) Collaborating and communicating; (3) Fostering mutual respect and trust; (4) Honoring individuality while maintaining perspective. 3 core areas for demonstrating collaboration and commitment were also defined: (1) Providing credible education; (2) Facilitating community connection; and (3) Delivering support.
Implementation of key learnings consisted of:
Establishing an internal culture based on the patient ally mindset
Creating a cross-functional working group to establish meaningful strategies and aligned approaches based on actionable insights from patients and HCPs
Founding a “Red Cell Revolution™” group of patients, caregivers and HCPs across three HHA indications (PK deficiency, Thalassemia and Sickle Cell Disease) and Agios representatives to define and collaborate on shared issues within the HHA community
Measurement of patient engagement activities to monitor the intended positive impact on the patient community
Conclusion: Inception of the Patient Vision Project stemmed from a growing awareness of the importance of the patient voice-understanding how patients want to be engaged-and a vision to create a transparent mechanism that supports industry partnerships with patients to improve the drug development process. Evaluating patient engagement from industry, HCPs, and patient perspectives enabled the strategic alignment and shaping of a vision for effective partnership. In addition to providing a path forward to the participants of the Project, this initiative challenges industry standards and provides a blueprint to other companies with a common goal of understanding patient needs to ultimately reduce disease burden and improve patient outcomes.
Disclosures
Andemariam:Sanofi Genzyme: Consultancy; NovoNordisk: Consultancy; Vertex: Consultancy; PCORI: Research Funding; HRSA: Research Funding; Hemanext: Consultancy, Research Funding; Agios: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Research Funding; GSK: Consultancy; Global Blood Therapeutics: Consultancy, Research Funding; American Society of Hematology: Research Funding; Bluebird: Consultancy; Forma Therapeutics: Consultancy, Research Funding; Afimmune: Consultancy; Emmaus: Consultancy; Connecticut Department of Public Health: Research Funding; Accordant: Consultancy. Schryver:Agios: Consultancy, Honoraria. Blaylark:Agios: Consultancy, Honoraria. Colasanti:Agios: Consultancy, Honoraria. Shah:Vertex: Consultancy; Global Blood Therapeutics/Pfizer: Consultancy, Research Funding, Speakers Bureau; Bluebird bio: Consultancy; Alexion Pharmaceuticals: Speakers Bureau; Agios Pharmaceuticals: Consultancy; Forma: Consultancy. Jonassaint:Agios: Consultancy, Honoraria; Expressive Painimation: Current Employment, Current equity holder in private company. Shah:Agios: Consultancy, Honoraria. Trimnell:Vertex: Honoraria; Agios Pharmaceuticals: Consultancy; Novo Nordisk: Consultancy; Pfizer: Consultancy, Honoraria; Bluebird bio: Honoraria; Novartis: Consultancy; Graphite Bio: Consultancy. Woolford:Agios Pharmaceuticals: Consultancy, Honoraria. Davis:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Gallagher:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Zaidi:Agios Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. John:Agios: Current Employment, Current equity holder in publicly-traded company. Sheth:Bristol Myers Squibb/ Celegene: Consultancy, Other: Travel support, Research Funding; Fulcrum: Consultancy; Chiesi: Consultancy; CRISPR: Membership on an entity's Board of Directors or advisory committees; Bluebird bio: Consultancy, Other: Travel support; Agios: Consultancy, Other: Travel support, Research Funding; Vertex Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal